Lewis acids and bases
| Acids and bases |
|---|
| Acid dissociation constant Acid-base extraction Acid-base reaction Dissociation constant Acidity function Buffer solutions pH Proton affinity Self-ionization of water |
| Acid types |
| Brønsted · Lewis · Mineral Organic · Strong Superacids · Weak |
| Base types |
| Brønsted · Lewis · Organic Strong · Superbases Non-nucleophilic · Weak |
A Lewis acid, A, is a chemical substance that can accept a pair of electrons from a Lewis base, B, that acts as an electron-pair donor, forming an adduct, AB as given by the following:
Gilbert N. Lewis proposed this definition, which is based on chemical bonding theory, in 1923.[1] The Brønsted-Lowry acid-base theory was published in the same year. The two theories are distinct but complementary to each other as a Lewis base is also a Brønsted-Lowry base, but a Lewis acid need not be a Brønsted-Lowry acid.
The classification into hard and soft acids and bases (HSAB theory) followed in 1963. The strength of Lewis acid-base interactions, as measured by the standard enthalpy of formation of an adduct can be predicted by the Drago-Wayland two-parameter equation.
Contents |
History
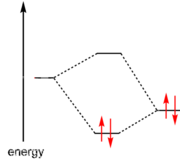
Lewis had suggested in 1916 that two atoms are held together in a chemical bond by sharing a pair of electrons. When each atom contributed one electron to the bond it was called a covalent bond. When both electrons come from one of the atoms it was called a dative covalent bond or coordinate bond. The distinction is not clear-cut as the diagram at the right shows; although the ammonia molecule donates a pair of electrons to the hydrogen ion, the identity of the electrons is lost in the ammonium ion that is formed. Nevertheless, Lewis suggested that an electron-pair donor be classified as a base and an electron-pair acceptor be classified as acid.
The modern definition of a Lewis acid is an atomic or molecular species that has an empty atomic or molecular orbital of low energy (LUMO) that can accommodate a pair of electrons, as illustrated in the molecular orbital diagram at the right.
Comparison with Brønsted-Lowry theory
A Lewis base is often a Brønsted-Lowry base as it can donate a pair of electrons to a proton; the proton is a Lewis acid as it can accept a pair of electrons. The conjugate base of a Brønsted-Lowry acid is also a Lewis base as loss of a proton from the acid leaves those electrons which were used for the A—H bond as a lone pair on the conjugate base. However, a Lewis base can be very difficult to protonate, yet still react with a Lewis acid. For example, carbon monoxide is a very weak Brønsted-Lowry base but it forms a strong adduct with BF3.
In another comparison of Lewis and Brønsted-Lowry acidity by Brown and Kanner,[2] 2,6-di-t-butylpyridine reacts to form the hydrochloride salt with HCl but does not react with BF3. This example demonstrates that steric factors, in addition to electronic factors, play a role in determining the strength of the interaction between the bulky di-t-butylpyridine and tiny proton.
A Brønsted-Lowry acid is a proton giver, not an electron-pair acceptor.
Lewis Acids
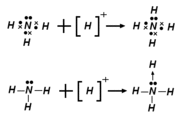
Some examples of reactions of Lewis acids, shown on the left.
- H+ + :NH3 → NH4+
- B2H6 + 2H− → 2BH4−
- BF3 + F− → BF4−
- Al2Cl6 + 2Cl− → 2AlCl4−
- AlF3 + 3F− → AlF63−
- SiF4 + 2F− → SiF62−
- PCl5 + Cl− → PCl6−
- SF4 + F− → SF5−
- Metal ions forming solvates, such as [Mg(H2O)6]2+, [Al(H2O)6]3+, etc. where the solvent is a Lewis base.
A typical example of a Lewis acid in action is in the Friedel-Crafts alkylation reaction. The key step is the acceptance by AlCl3 of a chloride ion lone-pair, forming AlCl4− and creating the strongly acidic, that is, electrophilic, carbonium ion.
- RCl +AlCl3 → R+ + AlCl4−
Lewis Bases
A Lewis base is an atomic or molecular species that has a lone pair of electrons in the HOMO. Typical examples are
- compounds of N, P, As, Sb and Bi in oxidation state 3
- compounds of O, S, Se and Te in oxidation state 2, including water, ethers, ketones, sulphoxides
- molecules like carbon monoxide
An easy way to remember this concept is that nearly all of the compounds formed by the transition elements are coordination compounds, wherein the metal or metal ion is a Lewis acid and the ligands are Lewis bases.
Hard and soft classification
Considerations concerning the strength of acid base adducts lead R.G. Pearson to propose, in 1963, the classification of both acids and bases into hard and soft. Within each category he established an order of binding strengths such as
- hard acids: R3P << R3N, R2S << R2O
- soft acids: R2O << R3N, R2S << R3P
For example, an amine will displace phosphine from the adduct with the acid BF3. In the same way, bases could be classified. For example, bases donating a lone pair from an oxygen atom are harder than bases donating through a nitrogen atom. Although the classification was never quantified it proved to be very useful in predicting the strength of adduct formation, using the key concepts
- hard acid — hard base interactions are stronger than hard acid — soft base or soft acid — hard base interactions.
- soft acid — soft base interactions are stronger than soft acid — hard base or hard acid — soft base interactions.
Later investigation of the thermodynamics of the interaction suggested that hard—hard interactions are enthalpy favored, whereas soft—soft are entropy favored.
References
- ↑ Miessler, L. M., Tar, D. A., (1991) p166 - Table of discoveries attributes the date of publication/release for the Lewis theory as 1923.
- ↑ Brown HC and Kanner B. "Preparation and Reactions of 2,6-Di-t-butylpyridine and Related hindered Bases. A case of Steric Hindrance toward the Proton." J. Am. Chem. Soc. 88, 986 (1966).
Further reading
- Jensen, W.B. (1980). The Lewis acid-base concepts : an overview. New York: Wiley. ISBN 0471039020.
- Yamamoto, Hisashi (1999). Lewis acid reagents : a practical approach. New York: Oxford University Press. ISBN 0198500998.
See also
- acid
- base
- Acid-base reaction
- Brønsted-Lowry acid-base theory
- Chiral Lewis Acid